A preprint, an unpublished non-peer reviewed study, from Public Health England (PHE) looks at the effectiveness of COVID-19 vaccines against the B.1.617.2 variant first identified in India.
Prof Paul Hunter, Professor in Medicine, UEA, said:
“The first thing to say is that are is still relatively few cases of B.1.617.2 compared to B.1.1.7 in the analysis with corresponding wide confidence intervals. The stated effectiveness of the vaccines in the preprint by Bernal are low even against the Kent variant B.1.1.7 compared to reports in the PHE vaccine surveillance report. The vaccine surveillance report states the effectives of a single dose of either vaccine (Oxford or Pfizer) as being in the range 55-70% with High confidence and after two doses in the range 85 to 90% with medium or for Oxford low confidence. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/988193/Vaccine_surveillance_report_-_week_20.pdf
“By contrast the Bernal pre-print gives estimates of the effectiveness after a single dose of either vaccine against B.1.1.7 as 51.1% (95%CI: 47.3 to 54.7) and for B.1.617.2 as just 33.5% (95%CI: 20.6 to 44.3). After the second doses of the Pfizer vaccine the effectiveness against B.1.1.7 was 93.4% (95%CI: 90.4 to 95.5) and 87.9% (95%CI: 78.2 to 93.2) against B.1.617.2. With the Oxford 2 dose vaccine effectiveness reduced from 66.1% (95% CI: 54.0 to 75.0) with B.1.1.7 to 59.8% (95%CI: 28.9 to 77.3) with B.1.617.2.
“As would be expected the India 2 is more resistant to vaccine given that it still has one escape mutation, though the two-dose regimen will still be effective and with the Pfizer vaccine being highly effective. It is too early to be very sure of the effectiveness of the two dose Oxford vaccine though if its effectiveness is only 60% effective then this would be disappointing especially for reducing transmission.
“Whilst much discussion about whether new variants are more resistant to vaccines or are more infectious the reality is often a combination of the two The combination of a modest increase in infectiousness with a modest increase in immune escape as seems to be the case here would be enough to increase the chance that the new variant would spread widely even with relatively high vaccine coverage.
“But what is clear from this research is that the main thing we can do to reduce the spread of this variant is to ensure that we get our second dose of vaccine whatever vaccine we had for our first injection. Although this study did not look at the protective effect of a previous natural infection, we do know that such natural infections do not provide complete protection at about 80% and maybe lower in older age groups https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00575-4/fulltext. Given that the Indian variant is able to resist vaccine immunity to some extent it is also likely to resist immunity to some extent from natural infections as well. Therefore, it is still really important to take up the offer of immunization even if you know you have had a prior infection. Although it is still a little early to be certain I think this evidence increases the likelihood that booster doses will need to be offered in the autumn.”
Dr Peter English, Retired Consultant in Communicable Disease Control, Former Editor of Vaccines in Practice, Immediate past Chair of the BMA Public Health Medicine Committee, said:
“The preprint’s introduction provides a useful summary of where we were at with the research into vaccine effectiveness against different SARS-CoV-2 variants prior to this research.
“The authors used two approaches to estimate the effect of vaccination on the so-called India variant – specifically, the B.1.617.2 variant (also referred to by PHE as VOC-21APR-022).
“The first approach was a test negative case control (TNCC) design, in which people who tested positive for either the B.1.617.2 variant or the B.1.1.7 variant were matched a person who was similar to them (a “control”), and the vaccination status of the cases and controls was compared.
“The second approach was to compare the proportion of cases with either of the above variants in people who had, or had not been vaccinated at least three weeks previously.
“They found that after a single dose of vaccine, the effectiveness of vaccines against the B.1.617.2 variant was not quite as good as against the B.1.1.7 variant – it was about 80% as effective. But there was very little reduction in effectiveness against symptomatic disease after two doses, as shown in the table below.
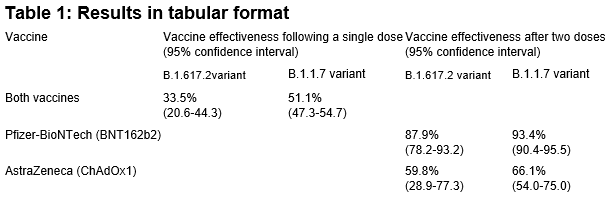
“The authors propose that these findings support giving the second dose of vaccine earlier in areas where the B.617.2 variant is known to be circulating, and may thus explain the policy being implemented in parts of the UK at present.”
Prof Adam Finn, Professor of Paediatrics, University of Bristol, said:
“These important data from PHE give us a first look at how the effectiveness of the two vaccines we have used the most so far holds up against the B1.617.2 variant that is beginning to circulate in the UK. Overall the results are encouraging in that the vaccines are continuing to provide useful protection. However, protection after the first dose appears to be reduced to a potentially important degree. Accordingly public health authorities seeking to control outbreaks should not rely too much on acceleration or extension of first dose administration to younger age groups as the impact of this, as well as taking some time, may now be reduced. Timely administration of second doses, by contrast, ought to provide rapid valuable additional protection against this new strain. It is also important to appreciate that these results relate to symptomatic infection, most of which will have been mild. It remains the strong expectation that both vaccines will continue to provide a high level of protection against severe disease, especially after the second dose.”
Preprint (not a paper): ‘Effectiveness of COVID-19 vaccines against the B.1.617.2 variant’ by Jamie Lopez Bernal et al.
This work is not peer reviewed.
All our previous output on this subject can be seen at this weblink:
www.sciencemediacentre.org/tag/covid-19
Declared interests
Prof Adam Finn: “AF is an investigator in trials and studies of several COVID19 vaccines including Oxford-AZ, Pfizer, Janssen and Valneva and advises the UK government and the WHO on COVID19 and other vaccines. He receives no personal income for this work and is remunerated solely through his employment by the University of Bristol.”
Dr Peter English: “Until earlier this year I was a PHE employee, and some of the authors are former colleagues of mine. No other conflicts of interest to declare.”
None others received.